Excerpt ABVC Biopharma, Inc. a clinical stage biopharmaceutical company developing therapeutic solutions in oncology/hematology, CNS, and ophthalmology, today announced the submission of its Vitrargus Phase II study to the Australian Bellberry Human Research Ethics Committee (HREC). Article Summary If the submission is received, HREC approval will lead to a Clinical Trial Notification (CTN) submission to...
Excerpt The new BioCina vaccine is being manufactured in an Adelaide facility purchased by the Bridgewest group in 2020 from Pfizer, maker of a successful Covid-19 vaccine – though Pfizer’s vaccine targets only the original strain of the disease. Article Summary The vaccine is claimed to be easily adapted to new variants of Covid-19, making...
Excerpt Victoria’s Propanc Biopharma Inc. , a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the results from a small trial of just 18 rectal cancer patients in complete remission using an immunotherapy called dostarlimab are “impressive,” whilst acknowledging there’s more work to be done. Article Summary...
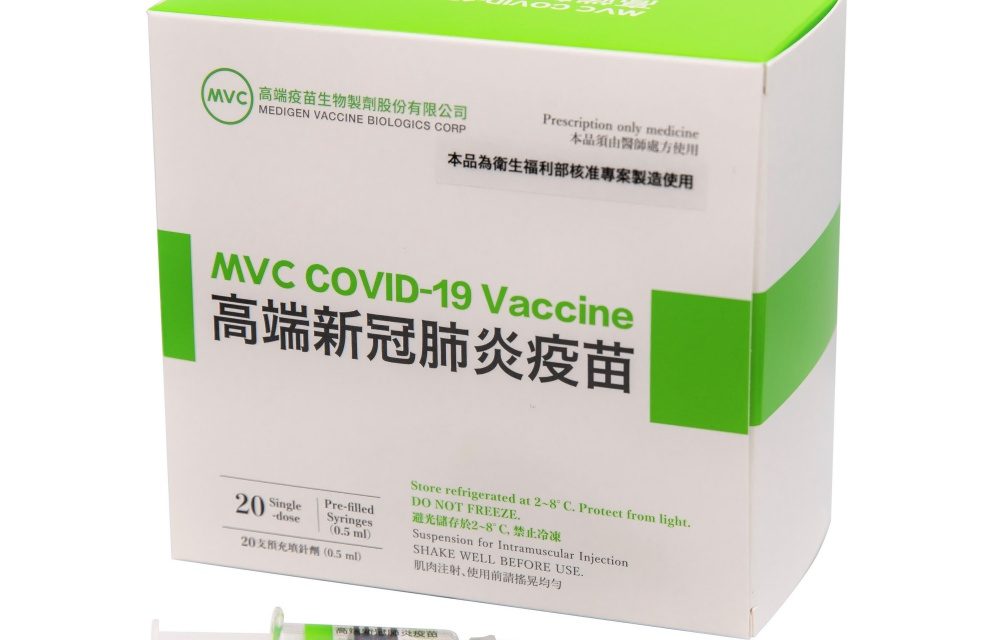
Excerpt Medigen Vaccine Biologics Corp (高端疫苗) has applied to the Australian medicines regulator for provisional approval of its COVID-19 vaccine, the pharmaceutical company said in a filing with the Taipei Exchange yesterday. Article Summary The company yesterday delivered the necessary documents to the Australian Therapeutic Goods Administration (TGA), but did not say when the regulator...