Asia Pacific Bioprocessing Facilities
$1,999.00
Featuring key Bioprocessing Facilities from China, Taiwan, Korea, Japan, India, SEA, and Australia! Asia-Pacific Bioprocessing Facilities report covers comprehensive information on parameters such as facility size, capacity, production technologies, types of biologics manufactured and much more. It provides readers a closer look into the facilities of Chugai, Hanmi, Daiichi Sankyo, Biocon (India & SEA), Beigene, Amgen (Singapore), GSK (Singapore) and more.
- Description
- Additional information
Description
- Overview
- Scope
- Benefits
- Key Questions Answered
- Data Methodology
- Table of Contents
- Figures & Tables
- User Access
The APAC Bioprocessing facility report features data analysis of key facilities manufacturing large molecules such as Monoclonal Antibodies, Recombinant Proteins, Cell Therapy, Bi-Specific Antibodies, Vaccines and more. The report covers the following regions: China, South Korea, Japan, Taiwan, India, Singapore, Australia and SEA. It sheds light on the facility design, bioprocessing technologies currently used across the manufacturing value chain (starting from cell-line development until fill & finish), plant capacity, facility type (single-use vs stainless steel) and facility investment.
IMAPAC Pte Ltd performed an analysis of the APAC bioprocessing facilities in the region to highlight the various trends of technologies used in the bioprocessing scene today. The report provides biopharma and CMOs the various types of features present in their competitors facilities to better understand the latter’s rationale behind building their facility in such a way.
The report presents insights from conversations obtained from senior representatives of biopharma companies on the following but not limited to:
- Project capacity & Production
- Biologics products manufactured at the site
- Scale of manufacturing (clinical or commercial)
- Bioprocessing employees in the facility
- Use of single-use vs stainless steels systems
- Bioprocessing tools, technologies & solutions employed
- Outsourcing plan
- Partnership & Collaboration
- Future facility expansion plans
The first of the report presents executive summary of the key findings captured in our study. It offers unique insights in relevance to bioprocessing systems and technologies adoption pattern in the Asian countries. It presents top insights into the growing interest of market players in technologies to suit the needs of their production of large molecules like vaccines, antibody fragments, matrix proteins and biosimilars.
The second section provides a general coverage of the report with further detailed analysis of bioprocessing facilities in each Asia-Pacific country including facility establishment, size, bioreactor used, processing technologies and facility investment. It includes a detailed expert poll & survey analysis of biopharma, big pharma and CMO with facilities in Asia such as their detailed profile, pilot and commercial batches run annually, their current focus in terms of cost saving opportunities and their technologies of interest. The section also provides exclusive insights from each biopharma facility from several countries including Greater China, India, Korea, Japan and South-East Asia.
The option to customize the report is available upon request to meet your data requirements which are not included in the report. The customized report is comparatively cost-effective and dependent on data requirements.
APAC Bioprocessing Facilities report features an extensive study of the APAC bioprocessing facilities operating in the region. The study includes an in-depth survey-based analysis, highlighting the capabilities of biopharma and contract manufacturing services providers engaged in the bioprocessing.
The report provides detailed overview on bioprocessing tools, equipment and technologies used by the biopharma, big pharma and CMOs in the facility with an objective to provide a view of competitive landscape and shed light on competitors clients.
Comprehensive regional analysis of the APAC Bioprocessing facilities covering facility information, plant design & capacity information, highlights of facilities and organizations covered in each region, highlights of facilities by types of products manufactured in Asia and analysis of cost-saving considerations in facilities.
Expert poll & survey analysis of bioprocessing facilities in each APAC geographies covering company overview, product pipeline, facility highlights, production capacity, bioprocessing technologies & equipment and insights into future expansion plans of biopharma and contract manufacturers in Asia.
A detailed overview of the bioprocessing facilities and landscape of companies engaged in this domain, based on several relevant parameters, such as list of bioprocessing organizations, in-house manufacturing capabilities, manufacturing facility establishment year, country, location, types of products manufactured, scale of operations (pre-clinical to commercialization), key features of facilities, bioprocessing services offered by company, plant capacity & batch size, facility size & processing technologies (batch/fed-batch/perfusion/continuous), expression systems (mammalian/microbial), name of technology or service providers, ongoing projects of the company, products in pipeline, upstream and downstream processing capabilities, fermentation scales, facility design, engineering, drug substance and product manufacturing, regulatory approvals & certifications accomplished by the organization, amount of investments made in the facility, partnerships & expansion plans of company.
IMAPAC Pte. Ltd. has released this report with an objective to support key industry stakeholders in co-development and other business-related partnerships, to plan future portfolio focus and to invest in research and technologies to fill the gaps identified. IMAPAC will continue tracking the developments in the industry and the implementation of its recommendations.
Asia Pacific Bioprocessing Facilities Report with 250+ page consists of 163 tables and 105 charts that are easy to comprehend. Read on to know more about the bioprocessing facilities in the Asia Pacific region, along with their future expansion plans. The report studies and analysis of key facilities throughout Asia Pacific. Discover exclusive insights from key bioprocessing facilities covering information such as facility establishment, size, key technologies and company profiles. This report lets you explore opportunities in the Asia-Pacific Bioprocessing space by providing deep-dive understanding of the bioprocessing landscape.
This report will assist you in critical decision making and help you to build informed strategies. The unique and detailed report will support your business plans and allow you to access and understand bioprocessing landscape better.
Through our report, you are more likely to stay ahead of your competitors and expand your knowledge and expertise in bioprocessing facilities. This report may potentially help your research, analyses, and strategic decisions. IMAPAC’s Report is intended for anyone who demands comprehensive and in-depth analyses for key APAC Bioprocessing Facilities.
- How many bioprocessing facilities are operating in Asia?
- Which products are manufactured in each facility in Asia?
- Which type of cell culture is used in each facility?
- What kind of bioreactors are adopted in the bioprocessing capacity?
- What is the scale of upstream manufacturing capacity of each facility?
- What are the current projects of biopharma organizations?
- What is the current focus of organizations in terms of cost saving involved in bioprocessing?
- In which phases of development does each organization currently have biopharmaceutical products?
- How many total batches did each facility run during the past 12 months for commercial production?
- What are the components of plant design and challenges faced in plant designing?
- What is the capacity of each manufacturing plant in litre?
- What are the future plans of Biopharma and CMOs in terms of facility expansion?
The data in this report is researched and validated using primary and secondary research. The data is collected through multiple scientific and non-scientific sources including live discussions with experts through IMAPAC’s events and research reports, company websites, press releases, publicly available information, regulatory database and many more.
- Preface
- Executive Summary
- Bioprocessing Facilities in APAC
- Report Coverage
- Regional Analysis of the Bioprocessing Facilities
- Facility Establishment vs Year of Operation
- Facility Size
- Bioreactor Type
- Expression System
- Process Technologies
- Facility Investment
3.3 Expert Poll & Survey Analysis 1: Biopharma, Big Pharma, CMO with Facilities in Asia
3.3.1 Respondent Profiles
3.3.2 Facility Type
3.3.3 Batches Run in 1 year (Pilot & Commercial)
3.3.4 When it comes to cost savings, what is your focus currently?
3.3.5 Facility expansion plans
3.3.6 Which Technologies are interesting for you?
3.4 Exclusive Insights from the Biopharma Facility
3.4.1 Kemwell Biopharma
3.4.2 Samsung Biologics
3.4.3 Kolon Life Science
3.4.4 Medipost
3.4.5 Binex
3.4.6 Eubiologics
3.4.7 United Biopharma
3.4.8 Celltrion
3.4.9 JHL Biotech Taiwan
3.4.10 AGC Biologics
3.4.11 Chugai Pharmaceuticals
3.4.12 Sumitomo Dainippon
3.4.13 Unicocell
3.5 Bioprocessing Facilities: Greater China
3.5.1 Akeso Pharmaceuticals
3.5.2 Beigene
3.5.3 Chime Biologics
3.5.4 Eirgenix
3.5.5 Innovent Biologics
3.5.6 JHL Biotech
3.5.7 Shanghai Junshi Biosciences Co Ltd
3.5.8 Medigen Vaccine Biologics Corporation
3.5.9 Mycenax
3.5.10 Pfizer
3.5.11 Pharmaessentia
3.5.12 Shanghai Henlius
3.5.13 Sinovac
3.5.14 Tonghua Dongbao
3.5.15 Tot Biopharma
3.5.16 United Biopharma
3.6 Bioprocessing Facilities in India
3.6.1 Aurobindo Pharma
3.6.2 Cpl Biologics
3.6.3 Enzene Biosciences Ltd
3.6.4 Intas Pharma
3.6.5 Kemwell Biopharma
3.6.6 Lupin
3.6.7 Serum Institute of India
3.6.8 Stelis Biopharma
3.6.9 Reliance Life Sciences
3.7 Bioprocessing Facilities in Korea
3.7.1 Aprogen Biologics
3.7.2 Binex
3.7.3 Celltrion
3.7.4 Eubiologics
3.7.5 Gc Pharma
3.7.6 Kolon Life Sciences
3.7.7 Medipost
3.7.8 Polus
3.7.9 Samsung Biologics
3.8 Bioprocessing Facilities in Japan
3.8.1 Astellas (Cell & Gene Therapy Plant)
3.8.2 Chugai Pharmaceuticals
3.8.3 Hitachi
3.8.4 Sumitomo Dainippon
3.9 Bioprocessing Facilities in South-East Asia
3.9.1 Abbvie
3.9.2 Amgen
3.9.3 Glaxosmithkline
3.9.4 Pt Kalbe
3.9.5 Siam Biosciences
3.9.6 Tcels
3.10 Bioprocessing Facilities in Australia
3.10.1 CSL Behring
3.10.2 Csiro
List of Tables
Table 1: Facility sizes of plants covered in APAC
Table 2: Expression systems used by facilities covered in APAC
Table 3: Process Technology used in the facilities
Table 4: Bioprocessing tools and equipment used in the manufacturing value chain
Table 5: Investment made for the facilities covered in APAC
Table 6: Highlights from APAC bioprocessing survey conducted
Table 7: Overview of the facilities featured in Greater China
Table 8: Akeso Pharmaceuticals Antibody Product Pipeline
Table 9: Zhongshan & Guangzhou Bioprocessing facilities overview of Akeso Pharmaceuticals
Table 10: Akeso Pharmaceuticals: Current & Future Bioreactor Capacity
Table 11: Process Technologies in Akeso’s Facilities
Table 12: Development milestones & achievements of Alphamab Oncology from year 2013 to 2020
Table 13: Suzhou Bioprocessing facility overview of Alphamab
Table 14: Alphamab’s Bi-specific Antibody Product Pipeline
Table 15: Current & future capacity of Alphamab Oncology
Table 16: Bioprocessing technologies, tools and equipment for Alphamab Oncology
Table 17: Beigene Immuno-oncology product pipeline (as of Jan 2020)
Table 18: Suzhou & Guangzhou Bioprocessing Facilities Overview of Beigene
Table 19: Summary of Bioprocessing Technologies: Beigene
Table 20: Facility highlights of Chime Biologics manufacturing plant
Table 21: Wuhan Facility Overview: Chime Biologics
Table 22: Different components of facility in Chime Biologics
Table 23: Details of bioprocessing equipment, model of equipment, & solution providers of Chime Biologics manufacturing facility
Table 24: Product pipelines of Eirgenix (as of Jan 2020)
Table 25: Facility Highlights of Hsinchu Biomedical Park, Zhubei: Eirgenix
Table 26: Bioprocessing equipment in Eirgenix’s mammalian cell culture facility
Table 27: Bioprocessing equipment in Eirgenix’s microbial fermentation facility
Table 28: Break-down of the bioprocessing facility in Eirgenix
Table 29: Regulatory approvals of Eirgenix from year 2005 to 2020
Table 30: Future expansion plans from stage 1, 2 and 3 of Eirgenix
Table 31: Product pipeline of Innovent Biologics (as of Jan 2020)
Table 32: Facility highlights of Innovent Biologics
Table 33: Facility overview and highlights of JHL Biotech
Table 34: Design features of JHL Biotech’s manufacturing facility
Table 35: Summary of bioprocessing equipment models and the solution providers associated with JHL Biotech manufacturing facility
Table 36: Facility overview of Junshi Biosciences
Table 37: Product pipelines of Medigen Vaccine Biologics Corporation
Table 38: Facility overview & highlights of Medigen Vaccine Biologics Corporation
Table 39: Highlights of the MVC’s facility
Table 40: Current vaccines manufactured in MVC facility
Table 41: Break-down of MVC facility
Table 42: Facility highlights & overview of Mycenax
Table 43: Product pipelines of Mycenax
Table 44: Product Pipeline: Mycenax
Table 45 Highlights of plant capacity: Mycenax
Table 46: Mycenax Biomanufacturing Plant Highlights
Table 47: Details of current aseptic filling line in Mycenax facility
Table 48: Technology platform in Mycenax’s bio-manufacturing facility
Table 49: Facility overview & highlights of Pfizer
Table 50: Details of different roles and partners in Pfizer’s manufacturing facility
Table 51: Product pipeline of Pharmaessentia
Table 52: Facility overview & highlights of Pharmaessentia
Table 53: Details of Pharmaessentia’s plant design and capacity as per EU/US standards
Table 54: Facility overview and highlight of Shanghai Henlius Biotech
Table 55: Facility overview and highlights of Sinovac
Table 56: Results of quality testing and regulatory approval of Sinovac’s manufacturing facilities
Table 57: Facility overview and highlights of Tonghua Dongbao
Table 58: Product pipeline of Tonghua Dongbao
Table 59: Facility overview & highlights of ToT biopharm
Table 60: Facilities overview and highlights of United Biopharma
Table 61: Overview of 3 plants of United Biopharma
Table 62: Types of bioreactors in Plant in Taiwan
Table 63: Types of bioreactors in Plant in Yangzhou
Table 64: Overview of R&D Center and Industry Scale Manufacturing Plant in Taiwan
Table 65: Overview of Pilot Scale Manufacturing Plant in Yangzhou
Table 66: Overview of bioprocessing equipment and technologies used for United Biopharma
Table 67: Overview of the equipment in the quality control laboratory of Taiwan plant
Table 68: Overview of facilities featured in India
Table 69: Facility Highlights of Aurobindo’s manufacturing plant
Table 70: Aurobindo’s pipeline information for their vaccines and biologics products
Table 71: Facility Highlights for CPL’s manufacturing plant in Gujarat
Table 72: Product Pipeline for CPL’s manufacturing facility
Table 73: Features of CPL’s Bulk Production and formulation facility
Table 74: Facility highlights of Enzene’s Manufacturing plants in Pune
Table 75: Facility highlights of Intas manufacturing facility
Table 76: Pipeline pf Plasma derived products
Table 77: Pipeline of Intas’s Biosimilars
Table 78: Progressive pipeline of products in the manufacturing facility
Table 79: Facility highlights of Kemwell’s manufacturing plant in Banglore
Table 80: Kemwell’s Product pipeline and their stage of development
Table 81: Type and batch sizes of bioreactor used in Kemwell’s facility
Table 82: Fill and Finish capabilities of Kemwell’s facility
Table 83: Flexibilty design checklist and features of Kemwell’s facility
Table 84: Equipment installed in Kemwell’s facility and their providers
Table 85: Facility highlights of Lupin’s manufacturing plant
Table 86: Lupin’s pipeline of products manufactured
Table 87: Equipment used in Lupin’s manufacturing facility
Table 88: Facility highlights of the Serum’s manufacturing plant in Pune
Table 89: Facility highlights on Stelis Biopharma’s Banglore facility
Table 90: Stelis Biopharma’s pipeline of Products and their stage of development
Table 91: Breakdown of Stelis Biopharma’s facility size into each space of the plant
Table 92: Process optimisation and capabilities for the two expression systems in Stelis Biopharma’s facility
Table 93: Drug substance manufacturing features and the respective expression systems in Stelis Biopharma’s facility
Table 94: Equipment used in Stelis Biopharma’s bioprocessing value chain and their provider
Table 95: Stelis Biopharma’s Fill and Finish Equipment and their features
Table 96: Facility highlights of Reliance Life Science’s manufacturing facility
Table 97: Reliance Life Science’s manufacturing plants and the products manufactured
Table 98: Overview of facilities featured in Korea
Table 99: Facility highlights of Osong Plant, South Korea: Aprogen
Table 100: Development of biosimilars in the pipeline of Aprogen
Table 101: Highlights of both Songdo and Osong manufacturing facilities: Binex, 2020
Table 102: Types of products manufactured for their clients
Table 103: Products currently in the R&D pipeline in Binex’s facility
Table 104: Number of Batches of Drug Substance and Drug Product Manufacturing completed in Binex’s facility
Table 105: Binex’s Songdo facility milestone timeline
Table 106: Binex’s Osong facility milestone timeline
Table 107: Various fill and finish systems employed and Binex’s capacities
Table 108: Process Technologies used in the various stages of development in Binex’s facility
Table 109: Facility Highlights of Celltrion’s three plants; Celltrion 2020
Table 110: Celltrion’s product pipeline and their stage of development
Table 111: Type and batch size of bioreactors used in Celltrion’s manufacturing facility
Table 112: Process technologies used in the bioprocessing value chain: Celltrion
Table 113 Features of Celltrion’s facility: Upstream to Packaging
Table 114: Facility Highlights on both plant 1 and plant 2; Eubiologics, 2020
Table 115: Product details of Euvichol and Euvichol-plus
Table 116: Type of bioreactor and batch sizes used in each of the expression systems in Eubiologic’s facility
Table 117: Equipment used in Eubiologic’s plant 1 and plant 2 in both the expression systems
Table 118: Bioprocessing equipment used across the Eubiologic’s value chain and their providers
Table 119: Facility Highlights of GC Lab cell
Table 120: Product Pipeline for Hwasun Facility
Table 121: Product pipeline for GC lab cell facility, as of May 2019
Table 122: Building layout of GC Pharma’s Hwasun Facility
Table 123: Facility highlights of Kolon Life Science’s B1 and B2 plants
Table 124: Facility Size of both plants in square ft: Kolon Lifesciences
Table 125: Equipment used in the bioprocessing value chain for B1 facility and their providers
Table 126: Equipment used in the bioprocessing value chain for B2 facility and their providers
Table 127: Facility highlights of Medipost’s manufacturing plant
Table 128: Checklist of their facility design and its flexibility
Table 129: Facility Highlights of Polus manufacturing plant, 2020
Table 130: Pipeline of products manufactured in Medipost’s facility: Polus
Table 131: Facility size in square metres: Polus
Table 132: Production capacity for drug substance and drug product manufacturing: Polus
Table 133: Facility highlights of Samsung Biologics three manufacturing plants: Samsung Biologics
Table 134: Bioreactor sizes and their respective stage of production for each of the manufacturing plants: Samsung Biologics
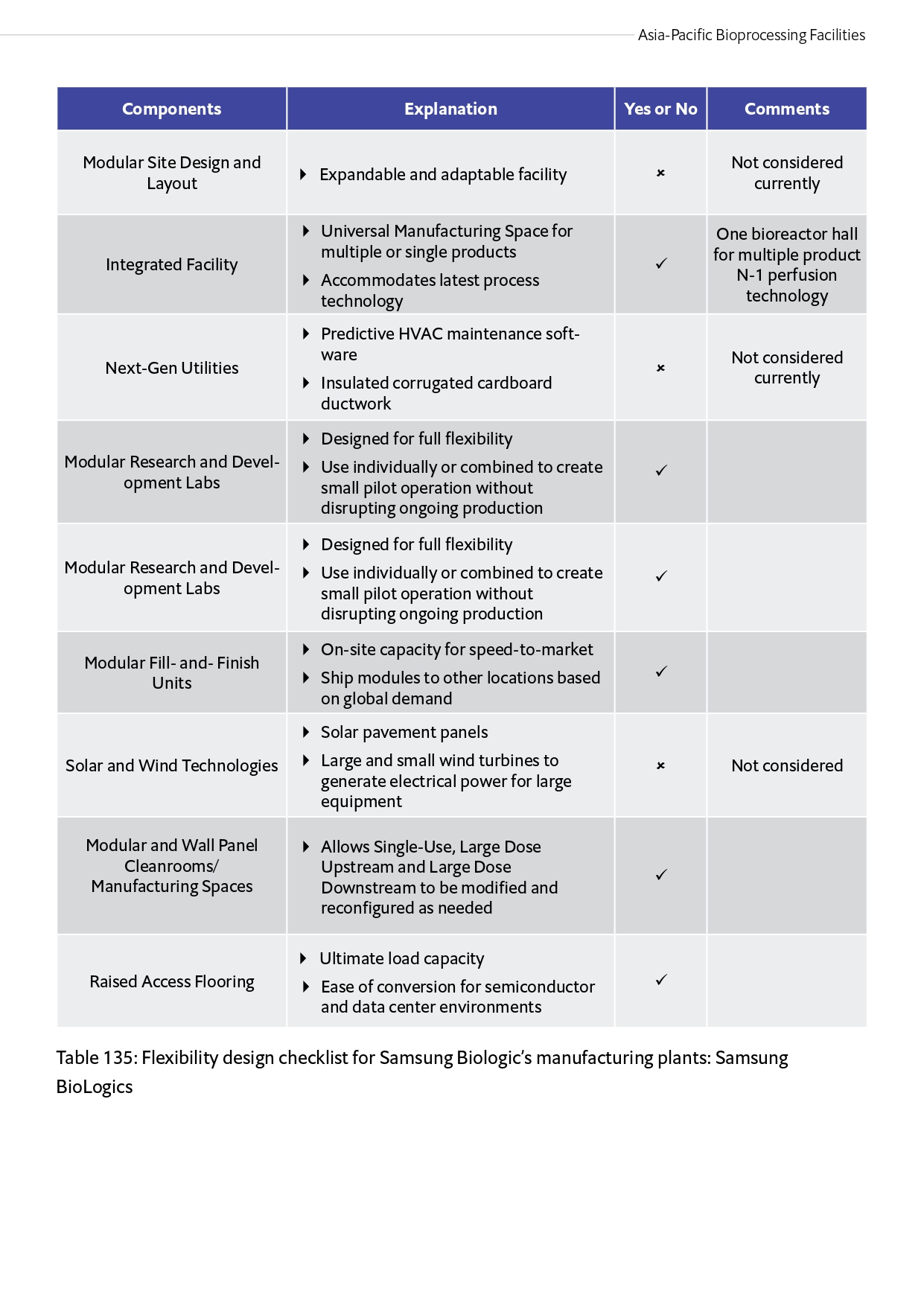
Table 135: Flexibility design checklist for Samsung Biologic’s manufacturing plants: Samsung Biologics
Table 136: Drug Product manufacturing for plant 1 and 2: Samsung Biologics
Table 137: Equipment used by their manufacturing plant and its provider: Samsung Biologics
Table 138: Overview of facilities featured in Japan
Table 139: Facility highlights of Astella’s cell therapy manufacturing plants
Table140: Facility Highlights of Ukima Plant1-3, Tokyo: Chugai Pharmaceuticals
Table 141: Facility Highlights of Utinosoma plant 1 and 2, Tokyo: Chugai Pharmaceuticals
Table 142: Chugai: Four products that have obtained Breakthrough Therapy Designation by the US FDA
Table 143: Pipeline of antibodies produced by Chugai and their stage of production
Table 144: Summary of the Chugai’s biologics manufacturing plants in Utsunomiya and Ukima, Japan
Table 145: Chugai: Comparison between Fed Batch process used in the rest of the plants and Perfusion Process used in UK3
Table 146: Hitachi: Facility Highlights of Yokohama facility in Japan: Hitachi Chemical Advanced Therapeutics
Table 147: Facility highlights of Sumitomo Dainippon’s plant
Table 148: Products under Sumitomo’s pipeline and their current development status, 2019
Table 149: iPS cell culture with automated cell culture equipment
Table 150: Overview on companies covered in SEA region
Table 151: Facility highlights for Abbvie’s manufacturing plant
Table 152: Equipment used in the bioprocessing value chain
Table 153: Next-Gen Biomanufacturing Facility, Amgen
Table 154: Facility Highlights of Amgen’s Tuas Plant, Singapore
Table 155: Features of Amgen’s flexible manufacturing plant
Table 156: GSK’s facility highlights in Tuas Biomedical Park
Table 157: Details of PT Kalbe’s Modular Facility Design
Table 158: Milestones Achieved by Siam Biosciences (2011-2020)
Table 159: CSL’s manufacturing facilities worldwide
Table 160: Facility highlights of Broadmeadows facility in Victoria, Australia
Table 161: Pipeline of products in CSL and their developmental stage
Table 162: The attributes and scope limits of downstream and upstream processing in the CSL’s biomanufacturing value chain
Table 163: Clayton manufacturing facility and the bioreactors used
List of Figures
Figure 1: Overall breakdown of the facilities in terms of their location, year of establishment and the features of the plant
Figure 2: Facility Establishment vs Year of Operation
Figure 3: Facility sizes of the manufacturing plants featured in the APAC region
Figure 4: Facilities with size ranging from 80,000sqm to over 200,000sqm
Figure 5: Percentage of bioreactor types employed
Figure 6: Regional distribution of bioreactor types used
Figure 7: SUS Use in China& Taiwan’s Bioprocessing Facilities
Figure 8: Stainless Steel System in Korea’s Bioprocessing Facilities
Figure 9: Percentage of expression systems employed
Figure 10: Percentages of the process technologies employed
Figure 11: Facility investment in APAC
Figure 12: Expression Systems in APAC Facilities
Figure 13: SUS Bioreactor: Process Development to Manufacturing
Figure 14: Batches Run by a Plant in 12 months (Commercial Production)
Figure 15: Batches Run by a Plant in 1 year (Pilot & Commercial)
Figure 16: Facility Cost-Saving Considerations & Need of the Hour
Figure 17: Key challenges faced by facility operators across Asia
Figure 18: Respondent Analysis: Expansion Plans
Figure 19: Process challenges faced by the industry
Figure 20: Technology need of the hour by ranking
Figure 21: Beigene’s KUBIO’s Biomanufacturing Facility, Guangzhou
Figure 23: Chime Biologics’s manufacturing facility in Wuhan BioLake Biotech Industry Development Zone
Figure 24: Bioprocessing equipment inside Chime Biologics’s manufacturing facility
Figure 25: Bioprocessing technologies inside Chime Biologics’s manufacturing facility
Figure 26: Bioprocessing equipment in Chime Biologics’s manufacturing facility
Figure 27: Bioprocessing technologies in Chime Biologics’s manufacturing facility
Figure 28: Main Line of Businesses: Eirgenix, Taiwan
Figure 29: Eirgenix’s commercial facility
Figure 30: Capacity of Eirgenix’s Hsnichu Biologics Plant
Figure 31: Milestones Achieved: Innovent Biologics
Figure 32: External look and interior design of Innovent Biologics manufacturing facility
Figure 33: Product pipeline of JHL Biotech (as of Jan 2020)
Figure 34: Process technologies of JHL manufacturing facility
Figure 35: Product pipelines of Shanghai Junshi Biosciences
Figure 36: The company structure of Medigen Biotech
Figure 37: Process Technologies Used by Mycenax
Figure 38: Bioprocessing technologies and equipment in Mycenax’s bioprocessing facility
Figure 39: Different technology platform in Mycenax’s bio-manufacturing facility
Figure 40: Regulatory approval timelines of Mycenax’s bio-processing facility
Figure 41: Facility scale-up and development of Pfizer
Figure 42: Number of resources working in different divisions in Taichung Plant
Figure 43: Milestones & Development timelines of Pharmaessentia from year 2003 to year 2019
Figure 44: Taichung PEG production area and filling plant
Figure 45: Process technologies in Pharmaessentia’s facility
Figure 46: Bioprocessing equipment in Pharmessentia’s Taichung plant
Figure 47: Regulatory approvals timelines of Pharmaessentia’s Taichung plant
Figure 48: Product pipeline of Shanghai Henlius Biotech
Figure 49: Changping Vaccine Production Center
Figure 50: Sinovac (Dalian) Vaccine Technology Co Ltd
Figure 51: Tangshan Yi’An Animal Vaccine Production Center
Figure 52: Beijing Headquarter
Figure 53: Product pipeline of TOT Biopharm
Figure 54: Flow chart for typical traditional process
Figure 55: Flow chart for PB Hybrid Technology
Figure 56: Facility scale-up and development timelines of United Biopharma
Figure 57: Exterior outlook of plant in Taiwan
Figure 58: Exterior outlook and bioprocessing equipment in plant in Yangzhou
Figure 59: Features of Upstream Processing, Downstream Processing and the expression platforms of the manufacturing facility
Figure 60: Process Technologies Employed in CPL’s facility
Figure 61: The red boxes above illustrate the disposable systems in CPL’s facility
Figure 62: cGMP Production Facility in MIDC, Chakan (Unit I) Pune, India
Figure 63: Manufacturing Plant in MIDC, Bhosati (Unit II), Pune India
Figure 64: Bioprocessing equipment in Mammalian Manufacturing Unit
Figure 65: Bioprocessing technologies in Mammalian Manufacturing Unit
Figure 66: Bioprocessing Technologies in E-Coli Manufacturing Unit
Figure 67: Stelis Biopharma’s facility expansion till FY 2020
Figure 68: Organisational experience in development, scale up and manufacturing in Stelis Biopharma
Figure 69: Overall Organisation Chart for Binex
Figure 70: Summary of their drug product manufacturing process flow
Figure 71: Process flow from cell line development to manufacturing
Figure 72: Product pipeline of Eubiologics, 2020
Figure 73: Overall layout of plant 1 in Chuncheon-si, Gangwon-do
Figure 74: Timeline of milestones achieved from 2016: Polus
Figure 75: Facility design from level 1 to 6
Figure 76: Astellas manufacturing plant in Toyoma Centre
Figure 77: Astellas manufacturing plant in Tsukuba
Figure 78: Chugai: Bioreactor columns and Purification lines in Chugai’s UK3 plant
Figure 79: Chugai: Box in Box reconfigurable Factory of UK3 plant
Figure 80: Chugai: Current Fed Batch Manufacturing Process employed in Uk1,2 and 3UT1,2
Figure 81: Chugai: Continuous Purification for UK3
Figure 82: Development of Chugai’s facility over the years
Figure 83: Yokohama Plant; Hitachi Chemical Advanced Therapeutics Solutions, 2020
Figure 84: Hitachi: Counter-Flow Centrifugation System
Figure 85: Hitachi Equipment found in the development room in Hitachi
Figure 86: Location of Sumitomo’s offices and plant in Japan
Figure 87: Cell Therapy Manufacturing plant in Osaka Prefecture, Sumitomo Dainippon
Figure 88: Closed system used by Sumitomo for cell therapy manufacturing
Figure 89: Automated cell- culturing equipment
Figure 90: Comparison between manual method and closed system
Figure 91: Sumitomo: The high-throughput cell sorter used in the manufacturing plant
Figure 92: Gigasort system, Cytonome/ST, LLC, Cell Sorter
Figure 93: Development process of ADCs
Figure 94: Amgen Facilities Operational Benefits generation facility Source: Biologics Manufacturing Asia (2019)
Figure 95: Comparison of Amgen Rhode Island Facility vs Amgen Singapore (Productivity vs Reduced Facility Size)
Figure 96: Closed processing system. Source: IMAPAC’s Biologics Manufacturing Asia Conference Singapore, 2018
Figure 97: Continuous perfusion culture in the next generation facility
Figure 98: Perfusion system and its impact on Volumetric productivity
Figure 99: Roller Bottle Technology used in PT Kalbio’s Manufacturing Plant; February 2019
Figure 100: Robotic Technology @ PT Kalbe’ Facility. Source: Biologics Manufacturing Asia 2019
Figure 101: Automated Chromatography Equipment: PT Kalbe, Source: Biologics Manufacturing Asia 2019 (February 2020)
Figure 102: Bioprocessing Equipment in Siam Biosciences, Thailand (April 2020)
Figure 103: R&D Manufacturing Suites of TCELS, April 2020
Figure 104: Design of the manufacturing suite: CSL Behring
Single – User Access
This license grants the right of use of the purchased report to a single recipient only. You may access the material on your computer, as and when required, for your own personal use.
Multi – User Access
Upto 3 users: This license grants the right of use of the purchased report by upto 3 users of the same firm/enterprise.
Corporate Access
Unlimited user access (Within your organization): This license entitles the buyer of the report to share, distribute the report (either full or in part) with other employees of the same firm/enterprise. The report may be accessed by any employee of the enterprise and there is no limit on the number of users.
Additional information
| User Access | Corporate Access, Multi – User Access, Single – User Access |
|---|


















Reviews
There are no reviews yet.