South-East Asia Bioprocessing Market
$1,999.00
Deep-dive into South-East Asia’s biopharma landscape with a comprehensive market & company analysis. Access in-depth market and company analysis of key biopharmaceutical companies in South-East Asia working on various biologics products such as vaccines, monoclonal antibodies, ADCs, biosimilars and cell therapies.
- Description
- Additional information
Description
- Overview
- Scope
- Benefits
- Key Questions Answered
- Data Methodology
- Table of Contents
- Figures & Tables
- User Access
South-East Asia Bioprocessing (SEA) Market Report features survey-based data analysis of Biopharma market in South-East Asia Region. The report covers competitive insights from top Biopharma, Biotech, Academic Institutes, and Contract Manufacturing Organizations (CMOs) involved in the development and manufacturing of large molecules such as Monoclonal Antibodies, Recombinant Proteins, Cell Therapy, Bi-Specific Antibodies, Vaccines, and more.
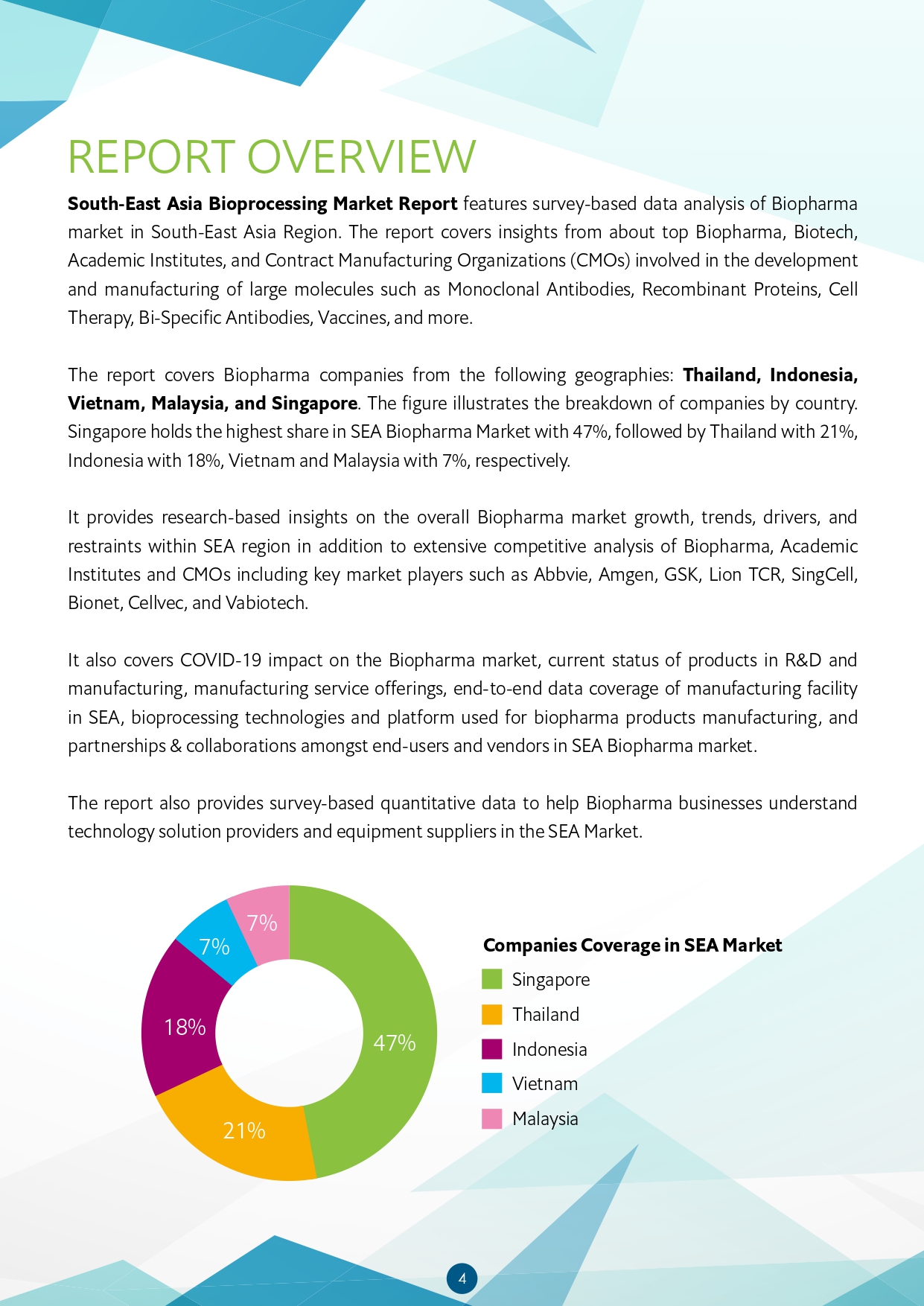
The first section of the report covers comprehensive market analysis of the South-East Asia bioprocessing including market size and projected growth. It includes analysis and research-based insights on the Biopharma market growth, trends, drivers, and restraints within SEA region. This section has scope of biopharma growth and opportunities in SEA region in addition to insights into top partnerships and collaborations in the industry. It also provides country wise analysis of Thailand, Indonesia, Singapore, Vietnam and Malaysia to provide insights into the biologics market growth in the region. It includes detailed analysis of market share by country. Singapore holds the highest share in SEA Biopharma Market with 47%, followed by Thailand with 21%, Indonesia with 18%, Vietnam and Malaysia with 7%, respectively.
The second section covers competitive landscape of Biopharma/Biotech companies, Academic Institutes and CMOs including Siam Biosciences, Tcels, Bio-Net Asia, Government Pharmaceutical Organisation, Kingen Biotech, Queen Saovabha Memorial Institute from Thailand. Singapore based players included are SingCell, BTI, Cell Research Corporation, Tessa Therapeutics, Dot Bio, Travecta Therapeutics, Prestige Biopharma, Esco Aster, Lion TCR. Malaysian companies included are Malaysian Vaccine Pharmaceuticals and National Institutes of Biotechnology Malaysia. Also studies Indonesian Biopharma companies such as Biofarma, PT Kalbe Global Biomedika, Daewoong Infion, Bandung Institute of Technology and Vietnamese companies such as Nanogen Pharmaceutical Biotechnology, Vietnam Academy of Science and Technology and Vabiotech.
It studies company’s products, service offerings, future investment plans, technology adoption, decision making process and COVID-19 impact on organisations among many other parameters.
The third section of the report provides analysis of SEA biopharma market based on the survey conducted by IMAPAC with companies including Kingen Biotech, The Government Pharmaceutical Organisation, Vabiotech, Vitech Development Co, Ltd, Malaysian Vaccines & Pharmaceuticals, National Institutes of Biotechnology Malaysia, PT. Combiphar Donga, PT. Kalbio Global Medika, Biofarma, Bandung Institute of Technology, Takeda, Cellvec, Bioprocessing Technology Institute. It reveals quantitative and qualitative findings to help Biopharma businesses understand technology solution providers and equipment suppliers in the SEA Market. It highlights the following parameters based on the questions covered in the survey
- Types of Biopharma Products Manufactured in SEA region
- Key Factors Influencing Supplier/Vendor Selection & Decision Making
- Competitive Landscape of Suppliers in SEA Region
- Customer Preferences of the Communication Channel
- COVID-19 Impact on Procurement Process and Regulations
- Future Plans of Biopharma, CMOs and Academic Institutes
This section also includes the list of respondents participated in the SEA Biopharma survey along with their responses.
The last section of the report features Sciex (Singapore).
The option to customize the report is available upon request to meet your data requirements for example any specific country or market data from SEA market. The customized report is comparatively cost-effective and dependent on data requirements.
As per the scope of the report, it studies the current and future landscape of the biologics market in the region. The report includes an in-depth study of SEA biopharma market, competitive analysis of companies and survey-based analysis of key players in the industry.
The report provides detailed overview on biopharma market growth and size. It covers market trends, challenges and opportunities in SEA region and offers competitive analysis-based insights for the market players with an objective to provide a view of competitive landscape and shed light on the overall biopharma market.
Some of the questions covered in the survey study are as following
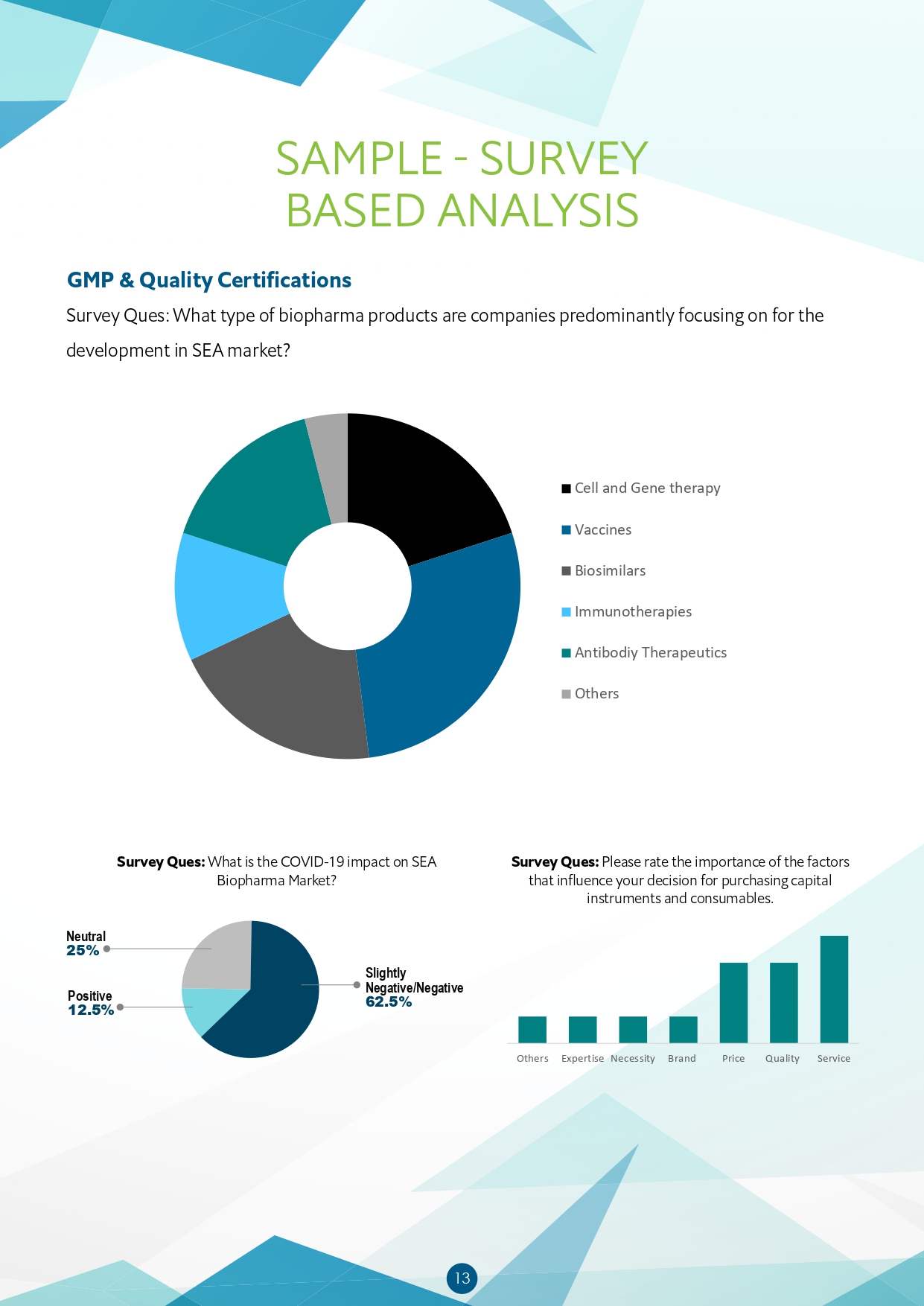
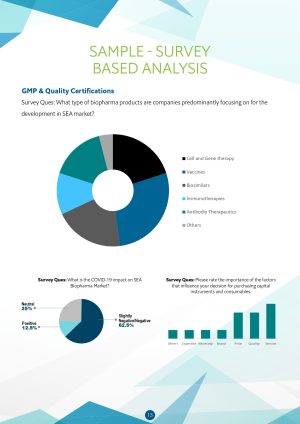
- What type of biopharma products are companies predominantly focusing on for development in SEA market?
- What is the COVID-19 impact on the SEA Biopharma Market?
- Rating the importance of the factors that influence decision for purchasing capital instruments and consumables.
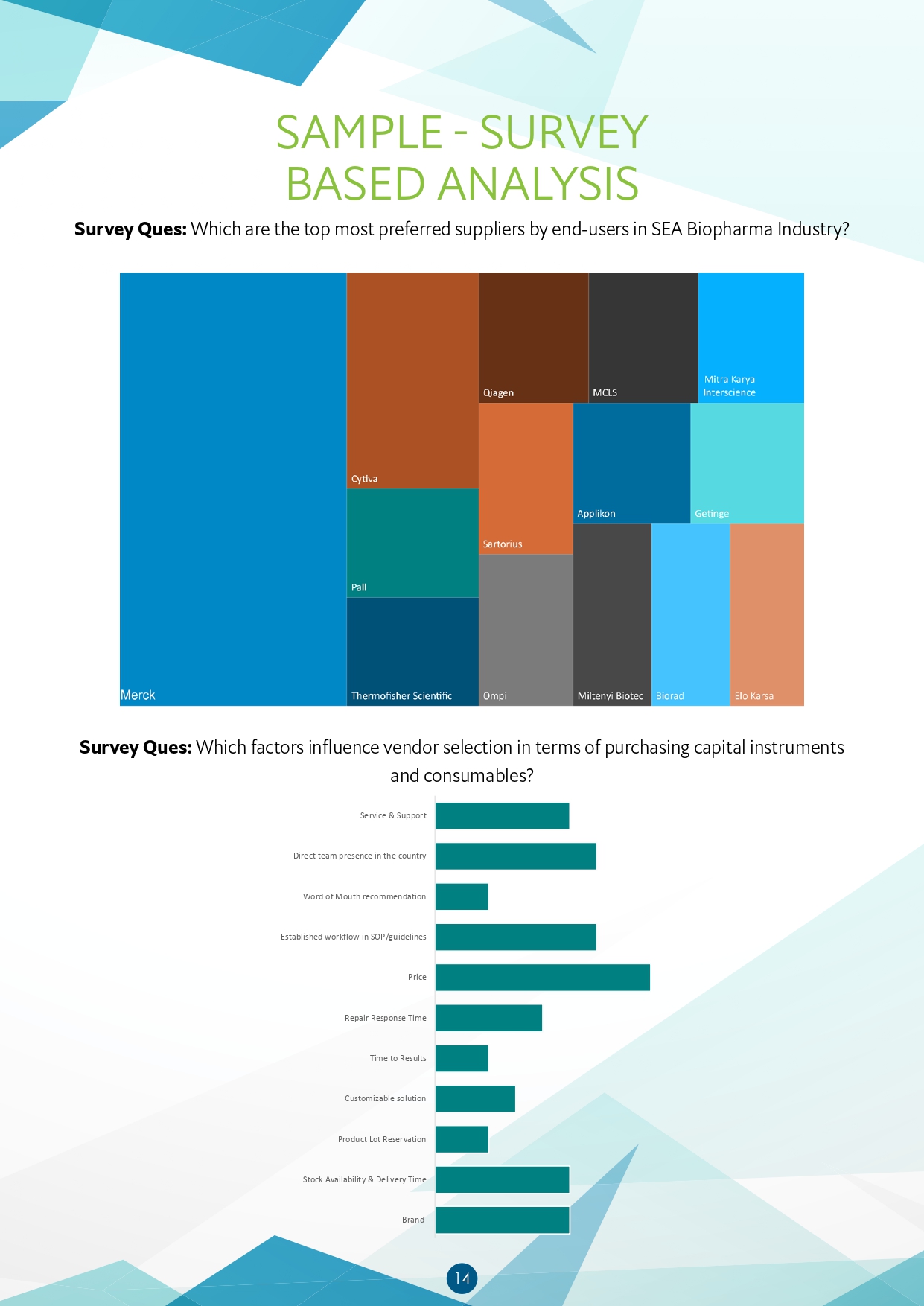
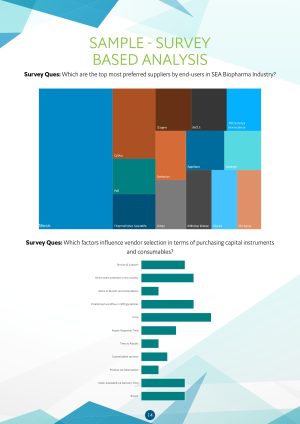
- Which are the top most preferred suppliers by end-users in SEA Biopharma Industry?
- Which factors influence vendor selection in terms of purchasing capital instruments
- and consumables?
To provide extensive information on market players in SEA region, the report includes company profiling, active products in pipeline with indication & development stage, manufacturing service offerings, details of manufacturing facility, production capacity & manufacturing process opted by biopharma companies, financial insights & investment plans, bioprocessing technologies and platform used for biologics manufacturing, and partnerships & collaborations amongst end-users and vendors in SEA Biopharma market.
IMAPAC Pte. Ltd. has released this report with an objective to support key industry stakeholders in co-development and other business-related partnerships, to plan future portfolio focus and to invest in research and technologies to fill the gaps identified. IMAPAC will continue tracking the developments in the industry and the implementation of its recommendations.
South-East Asia Bioprocessing Report with 110+ pages consist of 41 tables and 30 figures that are easy to comprehend. This report presents key details on the top Biopharma in the SEA Market, COVID1-9 Impact study, analyses data based on primary research around market growth & opportunities, industry trends, company profiles, service capabilities, manufacturing facilities, products in development, clients and investors, technology & solutions providers, financial insights, recent partnerships & collaborations, and exclusive insights obtained directly through interviews with experts.
This report is unique in that our charts and analyses figures are primarily based on data collected directly from key representatives from the Biopharma, Biotech, CMOs, and Academic Institutes in focus. This report lets you understand the competitive outlook of the biopharmaceutical industry in SEA region and assess any potential opportunity for business collaboration and investment.
Through our report, you are more likely to stay ahead of your competitors and expand your knowledge and expertise in bioprocessing facilities. This report may potentially help your research, analyses, and strategic decisions. IMAPAC’s Report is intended for anyone who demands comprehensive and in-depth analyses for key South-East Asia Bioprocessing Facilities.
- What is the overall growth and trend in SEA Biopharma Market?
- What has been the impact of COVID-19 on the market?
- What are the key drivers and restraints in each SEA geography?
- What are the key opportunities and threats faced by the biopharmaceutical players in this region?
- Which are the largest biopharmaceutical markets in the South-East Asia?
- Which is the fastest growing biopharma market?
- Who are the largest and top players in the SEA Biopharma Market?
- Where are biopharma manufacturing facilities located across SEA?
- What types of services are offered by companies in respect to the manufacturing of biopharma
- products?
- What are the key products manufactured in each facility by the scale of operations?
- Which key processing technology is used in facility?
- Which type of advanced or conventional technology is used in the facility with details on suppliers or
- vendors?
- Which are the key existing clients and partners with detailed insights on partnership activities?
- What are some of the GMP certifications and regulations followed in each plant?
- What are the future plans of Biopharma, CMOs and Academic Institutes in SEA?
- Which are the main focus areas in biopharma products development for Biopharma, CMOs and
- Academic Institutes?
- Enlist the factors affecting suppliers/vendors selection for equipment purchase?
- Who are the top preferred Biopharma suppliers in SEA Market based on the survey conducted by Imapac?
- What is the overall impact of COVID-19 on procurement process and regulations?
The data in this report is researched and validated using primary and secondary research. The data is collected through multiple scientific and non-scientific sources including live discussions with experts through IMAPAC’s events and research reports, company websites, press releases, publicly available information, regulatory database and many more.
- SEA Bioprocessing Market Analysis
1.1 Overall SEA Market Size and Project Growth
1.1.1 SEA Market Overview
1.1.2 Driving Factors in the SEA market
1.1.3 Limitations in the SEA Market
1.1.4 Partnerships and Collaborations
1.1.5 Scope of Growth
1.2 Country Wise Market Growth
1.2.1 Thailand Market Overview
1.2.2 Indonesian Market Overview
1.2.3 Singaporean Market Overview
1.2.4 Vietnamese Market Overview
1.2.5 Malaysian Market Overview
- SEA Based Biopharma/Biotech, Academic Institutes, CMOS Analysis
2.1 Thailand
2.1.1 Kingen Biotech
2.1.2 Bionet Asia
2.1.3 Siam Biosciences
2.1.4 Queen Saovabha Memorial Institute
2.1.5 The Government Pharmaceutical Organisation
2.1.6 Tcels
2.2 Indonesia
2.2.1 Biofarma
2.2.2 Pt Kalbe Global Biomedika
2.2.3 Daewoong Infion
2.2.4 Badung Institute Of oTechnology
2.3 Vietnam
2.3.1 Nanogen Pharmaceutical Biotechnology
2.3.2 Vabiotech
2.4 Malaysia
2.4.1 Malaysian Vaccine Pharmaceuticals
2.4.2 Alpha Biologics Sdn. Bhd. (Part Of Viropro Inc.)
2.4.3 Inno Biologics
2.4.4 Biocon Sdn. Bhd.
2.5 Singapore
2.5.1 Cellvec
2.5.2 Hummingbird Bioscience
2.5.3 2Tychan
2.5.4 Esco Aster
2.5.5 Prestige Biopharma
2.5.6 Lion TCR
2.5.7 Tessa Therapeutics
2.5.8 Dot Bio
2.5.9 Travecta Therapeutics
2.5.10 Cell Research Corporation
2.5.11 Singcell
2.5.12 Bioprocessing Technology Institute
2.5.13 Abbvie
2.5.14 Amgen
2.5.15 Glakosmithkline
2.5.16 Lonza Ag (Singapore)
- SEA Market Survey & Analysis
3.1. Kingen Biotech
3.2. The Government Pharmaceutical Organisation
3.3. Vabiotech
3.4. Vitech Development Co., Ltd
3.5. Malaysian Vaccines and Pharmaceuticals
3.6. National Institutes of Biotechnology Malaysia
3.7. Combiphar Donga
3.8. Kalbio Global Medika
3.9. Biofarma
3.10. Bandung Institute of Technology
3.11. Takeda
3.12. Cellvec
3.13. Bioprocessing Technology Institute
List of Tables
Table 1: Summary of the companies, products manufactured, and year established
Table 2: Thailand Biologics Market Growth with a CAGR of 12.5%: 2018-2025
Table 3: Future Expansion Plan of Kingen Biotech
Table 4: Equipment and solution providers of Kingen Biotech
Table 5: Active Pipeline of BioNet
Table 6: Characteristics of BioNet’s DNA vaccine platform
Table 7: Timeline of the production of Covigen; BioNet
Table 8: Active Pipeline of Siam Biosciences
Table 9: Production Area in QSMI
Table 10: Active pipeline of GPO
Table 11: Equipment and solution providers
Table 12: Future plans from 2020-2022; BioFarma
Table 13: Product pipeline; BioFarma
Table 14: Equipment and solution providers of BioFarma
Table 15: Facility highlights of KGM
Table 16: Future plans from 2020-2022; KGM
Table 17: Pipeline for PT Kalbe Global Medika
Table 18: Equipment and solution provider of KGM
Table 19: Product Pipeline of Daewoong Infion
Table 20: Product pipeline; Bandung Institute of Technology
Table 21: Equipment and solution provider of ITB
Table 22: Future plans from 2020-2022
Table 23: Active pipeline of Vabiotech
Table 24: Equipment and solution provider of Vabiotech
Table 25: Future plans from 2020-2022
Table 26: Active pipelines of MVP
Table 27: Equipment employed, and solution provider
Table 28: Product pipeline for Cellvec
Table 29: Equipment and solution provider of Cellvec
Table 30: Active pipeline of Hummingbird Bioscience
Table 31: Active pipeline of Tychan
Table 32: Phase 3 clinical trials of Covid-19 antibody
Table 33: Active pipeline; Travecta therapeutics
Table 34: Equipment and solution provider of Cell research corporation
Table 35: Product pipeline of BTI
Table 36: Equipment and solution provider of BTI
Table 37: Facility Highlights of Amgen’s Tuas Plant, Singapore
Table 38: Features of Amgen’s flexible manufacturing plant. generation facility
Table 39: GSK’s facility highlights in Tuas Biomedical Park
Table 40: Facility highlights of Lonza AG
Table 41: Sentiment analysis on companies’ Covid-19 budget
List of Figures
Figure 1: Market size of Thailand from 2018 to 2025
Figure 2: Snapshot of Thai Biologics companies
Figure 3: Snapshot of Indonesian biologics companies
Figure 4: Snapshot of Singaporean biologics companies
Figure 5: Milestone timeline; Daewoong Infion
Figure 6: Product pipeline of Vabiotech and their timelines
Figure 7: Process Flow & Purpose based segregation: Biologics Manufacturing Asia 2021
Figure 8: Process Flow & Purpose based segregation: Biologics Manufacturing Asia 2021 (2)
Figure 9: Process Flow & Purpose based segregation: Biologics Manufacturing Asia 2021 (3)
Figure 10: Steps in Fed-batch processing vs Continuous Processing (Biologics Manufacturing Asia 2021)
Figure 11: Continuous processing chromatography system (Biologics Manufacturing Asia 2021)
Figure 12: Preparative Crystallization + Pressure Filtration in Continuous Processing – Biologics Manufacturing Asia 2021
Figure 13: Product Pipeline and Stages of Development
Figure 14: IPSpheres Microcarrier Reprogramming Platform
Figure 15: COGS advantage of Singcell’s automated equipment
Figure 16: Cost savings with Singcell’s technology
Figure 17: Singcell’s automated bioreactor
Figure 18: Comparison of Autologous CAR-T Manufacturing Technologies
Figure 19: Next-Gen Biomanufacturing Facility, Amgen, Source: Biologics Manufacturing Aisa 2019
Figure 20: Amgen Facilities Operational Benefits
Figure 21 Comparison of Amgen Rhode Island Facility vs Amgen Singapore (Productivity vs Reduced Facility Size)
Figure 22: Closed processing system. Source: IMAPAC’s Biologics Manufacturing Asia Conference Singapore, 2018
Figure 23 Continuous perfusion culture in the next generation facility
Figure 24: Perfusion system and its impact on Volumetric productivity
Figure 25: Types of products manufactured
Figure 26: Factors influencing vendor selection
Figure 27: Top 5 voted parameters influencing buying decision
Figure 28: Decision makers for procurement
Figure 29: Various sources of information of companies
Figure 30: Voted best suppliers
Single – User Access
This license grants the right of use of the purchased report to a single recipient only. You may access the material on your computer, as and when required, for your own personal use.
Multi – User Access
Upto 3 users: This license grants the right of use of the purchased report by upto 3 users of the same firm/enterprise.
Corporate Access
Unlimited user access (Within your organization): This license entitles the buyer of the report to share, distribute the report (either full or in part) with other employees of the same firm/enterprise. The report may be accessed by any employee of the enterprise and there is no limit on the number of users.
Additional information
| User Access | Corporate Access, Multi – User Access, Single – User Access |
|---|










Reviews
There are no reviews yet.